Objective
To design and build a working prototype of an intervention device tester. The device consists of a test bed, support table, and force measurement unit. The device was built by two teams of four students with a medical device industry client. My team completed the test bed and table while the other group focused on the force measurement unit. The prototype was completed as a part of the McGill University Mechanical Engineering Capstone Project.
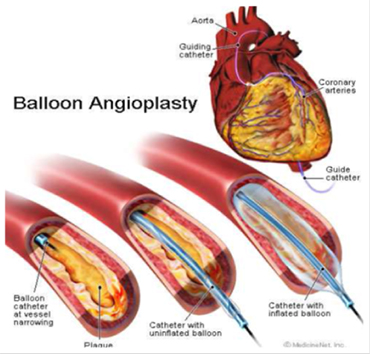
The device is used to test axial forces exherted on intravenous catheters after insertion into different circulatory system models for simulation. The test bed maintains a water temperature at 37°C to approximate human body temperature.
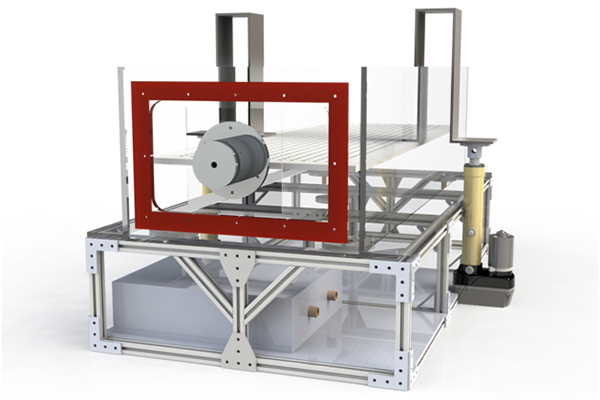
What is a catheter based intervention device?
A catheter based intervention device is a device designed to be brought subcutaneously (beneath the skin) through the circulatory system to a target treatment site. A primary benefit of catheter based interventions is the reduced invasiveness of the procedure when compared to direct surgery.
Why is an intervention device tester necessary?
A catheter based device must be stiff enough so that it can be pushed but also flexible so that it may navigate without causing damage to the blood vessels. The intervention device tester (IDT) advances and retracts a catheter through a model of a circulatory system, indirectly recording the forces exerted on the models walls by measuring axial forces at the base of the catheter.
Waterbath
The water bath is made from ½” acrylic which allows the tests to be fully observed and has reasonable thermal insulation. The strength and impact resistance was adequate for a clean lab environment. The bath was designed by the students but professionally built at Aquastyle Design.
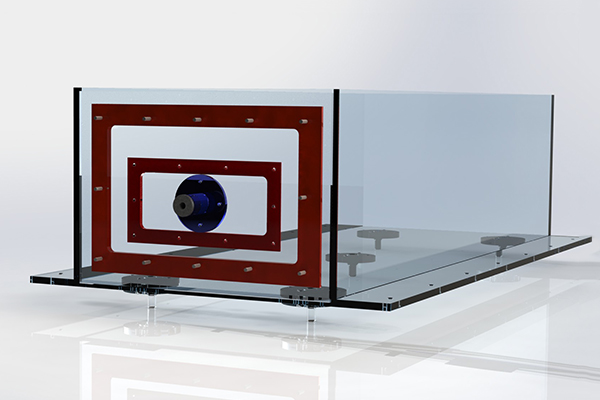
Submersed Model Platform
The simulation is performed with a variety of circulatory models of different sizes. The platform height can be adjusted with respect to the insertion point to accomodate for different size models. The platform is perforated with ¼” holes to both allow flow and to provide a plethora of model fixation points. The platform height is controlled by twin linear actuators adjusted by potentiometers with millimeter accuracy.


Insertion Port
The test models are desinged for zero-angle insertion. Therefore, the catheter must enter below the waterline of the test bed. A modular design was produced to accommodate different catheter sizes and insertion methods. The modularity comes in the form of a secondary plate which can be removed and altered. This allows for easy replacement and low cost modification if a catheter has unique introduction requirements.
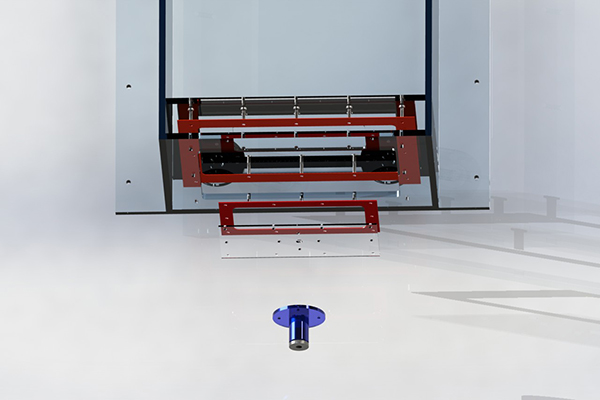
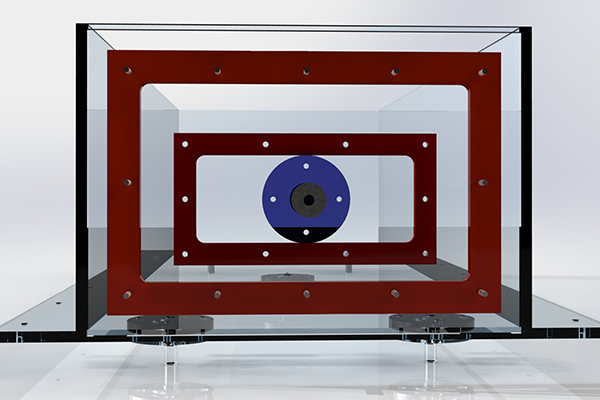
The Frame
For maximum modularity to accomodate any unforseen test criteria, the frame was made from 30mm T-slot profile extrusions. New additions can be quickly bolted on, and the frame can be completely taken apart.
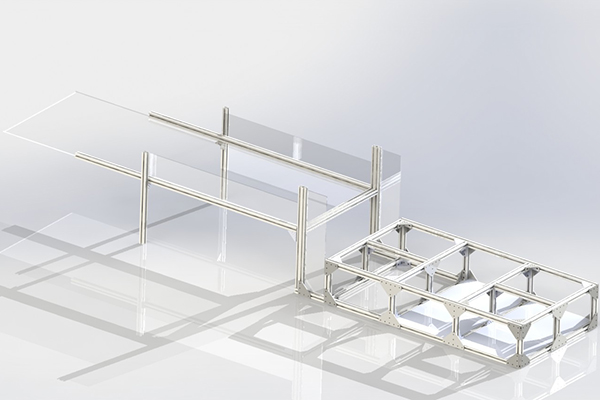
Temperature Control
The water is continuously recirculated in order to heat and maintain a temperature of 37°C. The water is pumped by an extended life seal-less pump and passes through a 1.2 KW Omega™ inline heater.


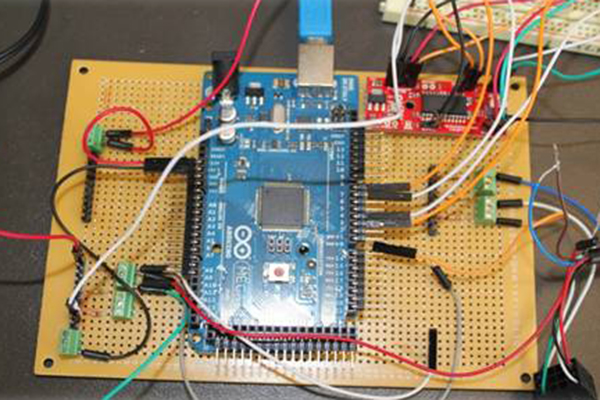
Electronic Control System
Two Arduino Mega 2560s interfaced with a LabVIEW™ DAQ control the entire Intervention Device Tester, connecting the temperature controller, the platform actuators, the pump, and the force measurement unit all in one hub. The temperature sensing and control is done by an Omega™ PID controller which can easily be removed and professionally tuned by a third party.
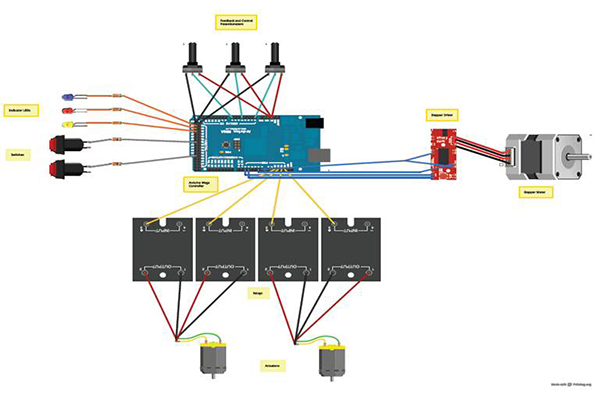
The Final Product
The final product was constructed at McGill University and put on display during the presentation period for all Mechanical Engineering Capstone Projects. It was then delivered to the client after the presentations were completed. Two of our group members (including me) went on to do respective internships for the company.
